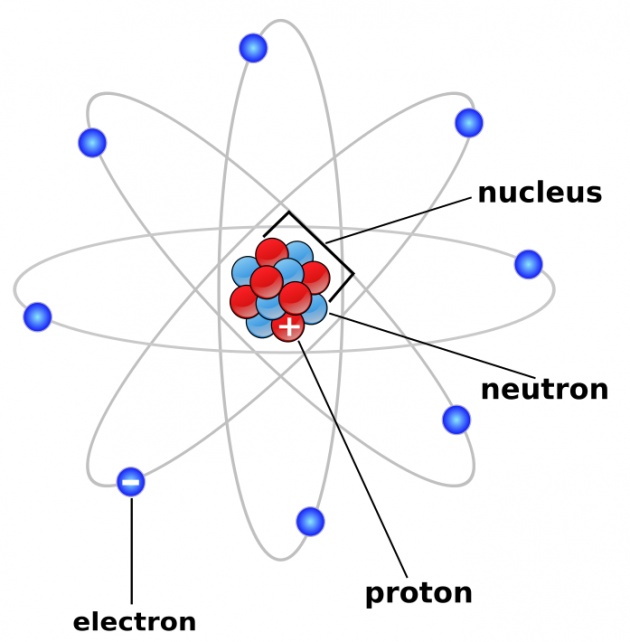
- As energy is required to remove an electron from the vicinity of the positively charged nucleus, therefore an electron must be be bound with negative energy and while going towards the nucleus, the energy of an electron decreases.
- It requires less energy to remove an electron from the outer shell as compared to the one in inner shell. we conclude that energy of an electron increases as it goes away from the nucleus.
- As Z (Atomic mass/ mass number) increases, the nuclear effective charge increases, and the binding energy as well.
- The energy level divides into sub-levels, in a sub-level the energy remain same for every electron however for the electrons in a energy level or a shell the energy may not be same.
- In ground sate all the electrons occupy the lowest possible energy states of an atom, however when an atom gets excited, the electrons jump to the higher energy-levels.
- With enough energy absorbed an electron leaves the vicinity of the atom.

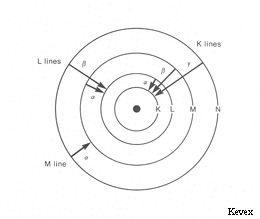
- Excitation or ionization may create the vacancy for an electron in one the inner shell. when that atom goes to the ground state, the vacancy is filled by the outer electrons. And energy is released in the form of EM waves.