بسم الله الرحمن الرحيم
Today I talk “chemical bond” first I can write what is chemical bond and its types in detail
Chemical bond
The attractive force which holds the atoms together in a compound is known as the Chemical bond .Therefore, to attain stability and to complete their outer most shell atoms with electrons combine chemically to form chemical bond
Types of chemical bond
There are four types of chemical bond
1) Ionic bond
2) Covalent bond
3) Co-ordinate Covalent bond or dative bond
4) Metallic bond
Ionic bond
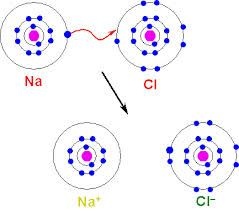
The electrostatic attraction between the oppositely charged species is called ionic bonds the attraction electron is transferred from one atom to other atoms as result the can produced two ions. For example the attraction between Sodium and Chlorine can making two ions Like Na+ and Cl -.Since they are oppositely charged chemical species they attract each other.
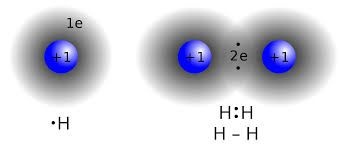
Covalent bond
Covalent bond can making by the mutual sharing of electrons between two atoms. Covalent bond may be single, double and tribal bond
In the covalent bond there are two types of bond
1) Polar bond
2) Non – polar bond
Polar bond
A polar covalent bond can making between two dissimilar atoms
Non – polar bond
A non- polar bond can making between two similar atoms
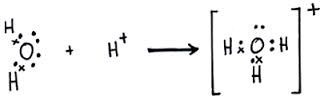
Co-ordinate Covalent bond or dative bond
Co-ordinate Covalent bond or dative bond is making when the shared pair of electrons are donated by only one atom
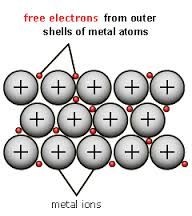
Metallic bond
Metallic bond exists in all metals. They are generally solids having high melting point and boiling points. They are good conductor because electricity and heat pass through in metals atoms. For example iron, copper, gold, silver etc there are many elements in the periodic table they are metals such as Na , Ca ,AL , Au, Ag, Pt etc . They have certain properties common. They are ductile, malleable, lustrous, and sonorous and they are good conductor of heat and electricity
Blog writer



