Specific heat of a substance is the amount of heat required to raise the temperature of 1 kg mass of that substance through I K.
Its formulae is c= delta Q /m (delta T)
When a body is heated its temperature increases.

IMPORTANCE:
Specific heat of water is 4200 J/kg/K and that of dry soil is 810 J/kg/K. As we know that specific heat of water is large than soil so the temperature of soil increases and decreases more rapidly than water. Temperature of soil increases five times more than water. That’s why the temperature of land rises or falls rapidly than sea. And during winter and summer the temperature of the places near sea are similar to each other it is also due to specific heat.

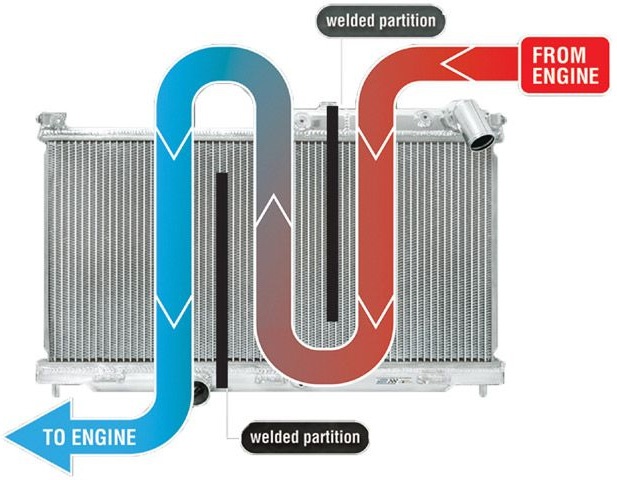
Water has large specific heat capacity and it is also useful for mankind as we can use it to store thermal energy. The cooling system of automobiles uses water to carry away undesirable current vitality. In an automobile large amount of heat is produced when its engine works at great speed and due to this heat its temperature goes on increases. The engine will stop if it not cooled down. Water circulates around engine and absorbs the heat to maintain the temperature of the automobile.