How to Protect Yourself from Common Bomb Radioactive Materials
(For tldr; go to summary).
Converting Uranium into Other Elements
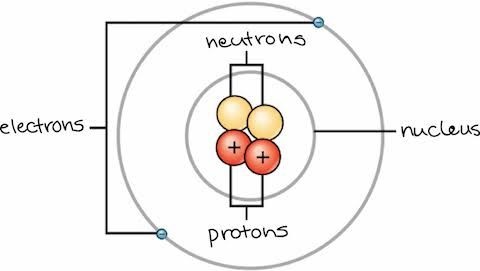
Photo credit: khanacademy.org
Particles in an atom (electrons, protons, neutrons) are held together by different types of forces in balance. One can weaken or strengthen these forces by either fission (splits nucleus) or fusion (combines nucleus) reactions. These reactions are used for nuclear power (eg: in engines or electricity).
Nuclear fission and fusion. Video credit: GRS | Deutschland via youtube.com
In nuclear fission, a very large element, such as uranium-235--used in fission bombs, undergoes reaction with a neutron to split into different types of smaller elements and other fission products such as radiation, Iodine-131, Strontium-90 and Cesium-137. Radioactive decay means an atom splits and emits radiation.
Understanding Radiation
Radiation (also called electromagnetic radiation) is all around us. Without it, life wouldn't thrive on earth. The sun is a good example: it emits light rays for plants and living beings to live. Other examples are radiation used in medicine and telecommunications. Without them, our way of living wouldn't be the same--it would make it primitive.
Electromagnetic (EM) radiation is the movement of energy - through space or a medium - composed of both electric and magnetic waves.
- from study.com
All radiation have this so-called packet of energy (photons) that can behave like waves (no "rest" mass) or particles (have mass) or both, simultaneously. If it, as an example, acts as waves, we have microwaves. If particles, the sun rays can excite plant cells in photosynthesis. Energy in photons might be weak (long wavelength) or strong (short wavelength).
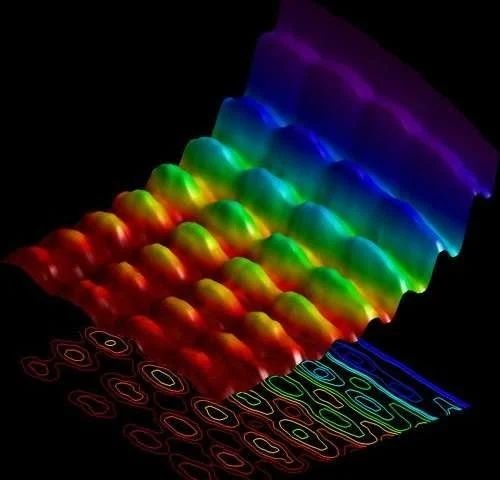
Image of photon as particle and wave. Photo credit: phys.org
Ray can be a shorter term for radiation.
Types of Radiation
There are 2 types of radiation: non-ionizing and ionizing. In non-ionizing radiation, energy emitted are weak that it can't completely remove electrons from an atom (eg.: radiowaves).
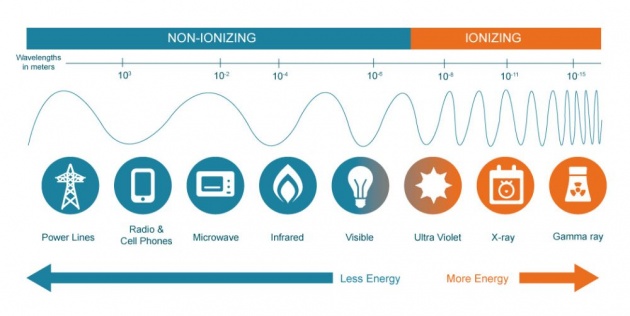
Photo credit: mirion.com
In ionizing radiation, the emitted rays can cause other atoms to loose electrons or neutrons, and become unstable and have charge (ions). Ionized rays come from already unstable atoms that have absorbed much energy and/or mass.
Types of Ionizing Radiation
There are 3 types of (direct) ionizing radiation: alpha, beta, and gamma radiation.
Video credit: GRS | Deutschland via youtube.com
Alpha Particles/ Radiation
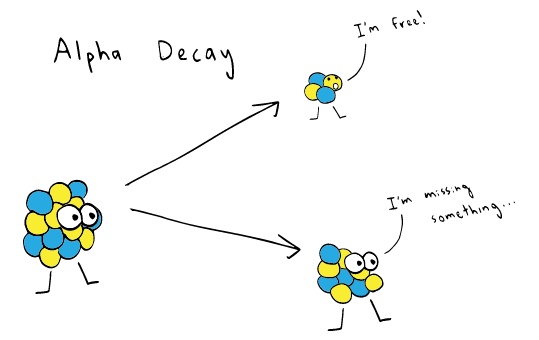
Photo credit: khanacademy.org
Alpha particles are formed when an unstable atom form one group of nucleus (mother nucleus), and another smaller group of protons and neutrons (daughter nucleus). This daughter nucleus have 2 protons and 2 neutrons, which is the alpha particle, and is similar to a Helium cation. Because these particles travel at a short distance and are larger than other solid molecules, a piece of paper can block their movement.
Alpha particles are innocuous but they're insidious if they enter our bodies. The best way is to cover bodily openings by masks, ear plugs and goggles.
Beta Particles/ Radiation
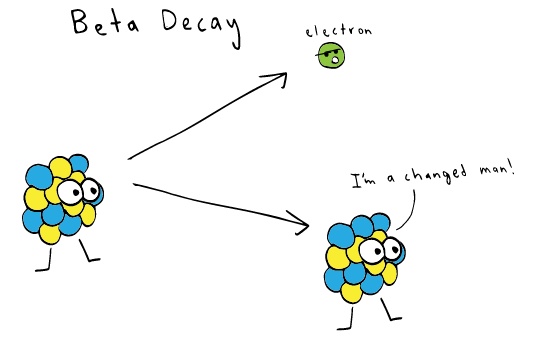
Photo credit: khanacademy.org
Beta particles are fast moving electrons or positrons emitted by unstable atoms. They are formed from converting neutron or proton into electron or positron, respectively, by high energy. Some beta particles can be stopped by proper clothing or simply by a tin foil. They are also harmless but can pose a threat if it gets inside the body, so ready your N95 masks.
Gamma Ray/ Radiation
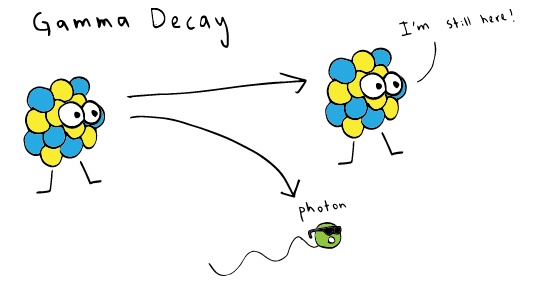
Photo credit: khanacademy.org
In a gamma ray, the atom retains all its subatomic particles, but these particles rearrange into low energy configuration, so the excess high energy (photon) gets released. These photons are what makes up gamma rays. Gamma radiation is the most lethal type of direct ionizing radiation because it penetrates in the deep layers of the skin. Our cells absorb this ray, where it destroys our DNA and makes us ill. It can be stopped by about 1.3ft of lead, 6.6ft of concrete or 13.1ft of water.
Common Harmful Radionuclides from Uranium Bombs
The Butterfly Killer: Iodine-131
Iodine-131 is composed of 78 neutrons and 53 protons, hence the name (sum of protons and neutrons). The thyroid gland, located at the throat, has a behavior wherein all iodine taken in by the body will be absorbed by it. Radioiodine is used in radiation therapy for hyperthyroidism in smaller doses, but a large dose poses instant cancerous threat in this butterfly-shaped gland.
Photo credit: nukepills.com
The best way to avoid absorption of Iodine-131 is by filling the thyroid with generally harmless iodine. For adults (19-40y/o) 130 mg of iodine is the dosage needed to fill it up, while for children (3-18, <70kg) it is 65mg. Do remember that Iodine-131 has a half-life of only about 8 days, so it's best to take the pill for about 1 week to minimize absorption.
The Bonebreaker: Strontium-90
Strontium-90 is a radioisotope that has a half-life of about 29 years. It has 52 neutrons and 38 protons, so the name. This radionuclide has similar characteristics with calcium: it enters the bones and "calcifies." The body cannot distinguish between calcium and Strontium-90 so it's very dangerous if you're exposed to it, because it emits radiation for a long time. Only 30% is absorbed by the bones, but it's still alarming because you can still have bone diseases.

Photo credit: amazon.com
Just like our thyroid glands, it can be remedied by taking the recommended dosage of what the bone absorbs: calcium. Calcium carbonate is effective in minimizing strontium-90's absorption by the body. CaCO3 contains 40% calcium so 2 1/2 500mg pills of CaCO3 (for 19-50y/o) is needed to take until the threat subsides (but its best to get calcium from uncontaminated food, like milk).
The Bloodhound: Cesium-137
Cesium-137 has a 30-year half-life. 82 neutrons and 55 protons comprise of this radioisotope. It can easily be dispersed through air and it quickly dissolves in water (vapors, rain, rivers, etc...) so it is highly dangerous. Luckily, cesium-137 is only artificially made; this radioisotope isn't found originally in nature.

Photo credit: mcguffmedical.com
Radiocesium behaves like potassium (an electrolyte) in the body, so it may affect the heart, soft tissues and some bodily fluids. It can cause bone marrow disease, like leukemia, and other forms of cancer. Prussian blue, or also known as ferric ferrocyanide, is used to counter absorption of cesium-137. Dosage varies for adults: either 1-3 grams/day or 10-12grams.
Summary
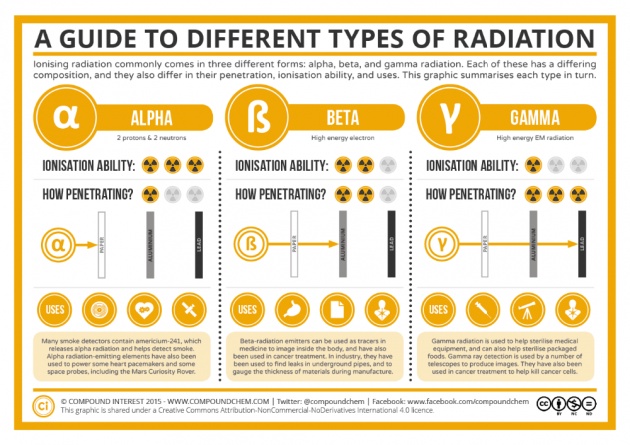
Photo credit: compoundchem.com
We need to protect ourselves from harmful radiation by covering our openings with either masks, plugs, clothes, and thick walls of concrete and/or lead. If we're exposed to Iodine-131, Strontium-90 and/or Cesium-137, it's our priority to take Potassium iodide, Calcium carbonate and/or Prussian blue, respectively, at recommended dosages. Understanding how things react and influence other things is helpful in preventing bad outcomes.
References:
http://teacher.nsrl.rochester.edu/phy122/Lecture_Notes/Chapter22/Chapter22.html
https://nuclear.duke-energy.com/2013/01/30/fission-vs-fusion-whats-the-difference
https://science.howstuffworks.com/radiation.htm
https://www.mirion.com/introduction-to-radiation-safety/what-is-radiation/
http://nuclearconnect.org/know-nuclear/science/protecting
https://en.m.wikipedia.org/wiki/Iodine-131
https://ronconte.wordpress.com/2011/03/14/potassium-iodide-use-in-radiation-emergencies/
https://en.m.wikipedia.org/wiki/Strontium-90
https://en.m.wikipedia.org/wiki/Caesium-137
https://www.remm.nlm.gov/prussianblue.htm
Disclaimer: Information stated in this blog post are for educational purposes only. Seek advise from an expert in the given field. Consult your physician for any medical advice. Writeup made by lapiz-lazuli.