Organic compounds have many characteristics and have a great importance in the field of chemistry. There are large number of organic compounds like, methane ethane propane, butane, hexane, heptane, octane. These compounds are placed in different series on the basis of physical and chemical properties.
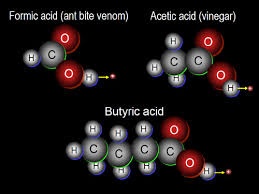
The most common series are hydrocarbon, alcohol, carboxylic acid,amines, carbonyl compound and ether. Common characteristics of organic compounds are that, organic compounds are covalent in nature. They are non polar and do not conduct electricity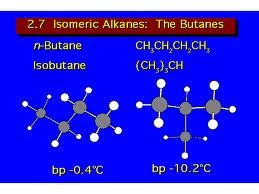
Organic compounds are insoluble in water because they are non polar but water is polar in nature and as we know that, polar salute dissolved in polar solvent and non polar salute dissolved in non polar solvent. So they are soluble in non polar solvent like benzene and ether.

Organic compounds have low melting and boiling point and are thermally unstable and decompose on the temperature of 500 degree centigrade. When the organic compound burn it give smoky flame. Organic compounds dissolved in benzene to form different products.

Organic compounds are used for the preparation of medicine. It is also used in the laboratory for the preparation of different chemicals.Organic compounds also have a great importance that it is used industries for the manufacturing of different products.



