In this blog i tell you that are we determine boiling point of solution or liquid .The temperature of a required at which the vapors pressure of liquid becomes equal to the atmospheric pressure, the liquid begins to boil at that time the temperature of liquid are called the boiling point of that liquid.
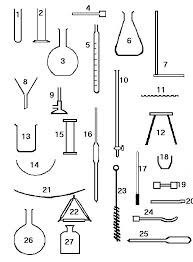
We know that a pure liquid always have a constant boiling point. For that experiment we need a tripod stand, beaker, thermometer, thin walled test tube , capillary tube , fusion tube, stirrer , iron stand, tripod and spirit lamp or burner.

In this experiment we use the alcohol as a liquid. We take a small amount of liquid in a thin walled test tube and place a capillary tube seed at about 1 centimeter from the law and of test tube and fix thermometer in it.
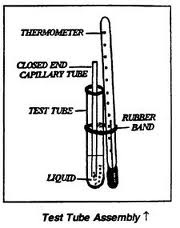
After that place the test tube in the beaker having water. Heat the the beaker and stir liquid constantly. After sometime the air bubbles rise from the lower end of capillary tube. Finally these bubbles become more and more according to the rising of temperature.
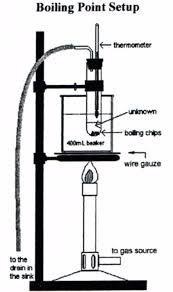
When the temperature become constant not the temperature and repeat this experiment 3 times and get the actual reading of that experiment .these readings are the boiling point of that liquid.