Noble Gases
Introduction of Noble Gases
Helium(He),Neon(Ne),Argon(Ar),Krypton(Kr),Xenon(Xe) and Radon (Ra) are the known as the noble gases or zero group elements.Their electronic configuration is ns2 np6 i.eight electrons in the outermost shell.As they have a stable structure,they do not show any tendency to gain or lose electron.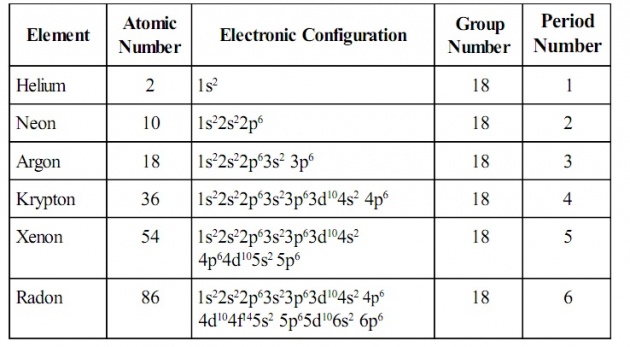 They are therefore,incapable of forming compounds under the ordinary conditions.
They are therefore,incapable of forming compounds under the ordinary conditions.
These elements are also called inert or noble gases because these are chemically inert and they only exist in the free state.They also have an another name i.e rare gases because they exist in very small quantities in the atmosphere.
Occurrence of Noble Gases in Nature
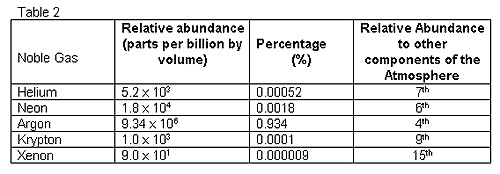
The Noble gases exist in minute quantity in the atmosphere.These gases can be isolated by fractional distillation of liquid air.The chief sources of noble gases are as under,
1.Air is the main source of all the inert gases except radon.
2.Helium can be obtained from natural gas wells.
3.The dissolved gases of certain spring waters contain considerable amounts of helium.
4.Helium is the 2nd most abundant element in the universe.
5.Alpha particles are doubly ionized helium atoms.
6.It is simple and economical to isolate the helium gas by liquification method.
7.Neon and Argon is obtained as a by product during liquification of air.
8.Traces of krypton are present in air.
9.Xenon is present in the atmosphere to a very small extent of 0.008 parts per million.It is obtained as a by-product during the liquification of air
10.Radon is the alpha decay product of the radium and exists in air in very small quantity.
11.All these gases are monoatomic.The boiling and melting points of elements are very low.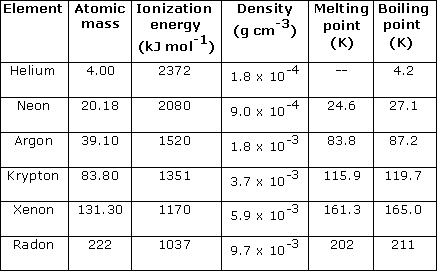
12.Helium has the lowest boiling point.
13.The ionization energy of noble gases is very high.
14.The major components of Xenon are oxides,fluorides and oxy fluorides.