The splitting of a heavy nucleus into two or more moderate size nuclei is called nuclear fission.A typical fission process of U-235 by capturing slow neutron is showed in the following pictures.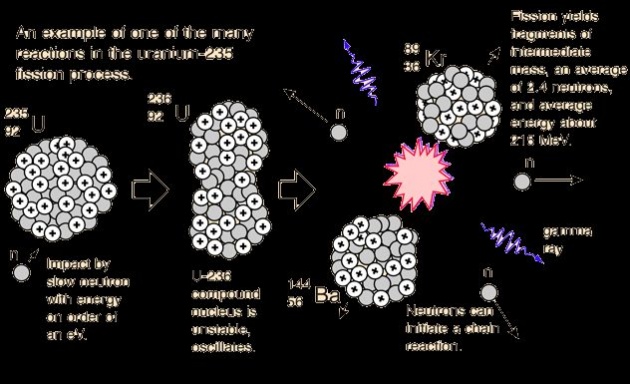
A slow neutron is absrbed by U-235 nucleus,it becomes unstable and change into U-236 which is said to be compound nucleus,U-236 then disintegrates into fission fragments barium and krypton
neutron+Uranium-235-------->{Uranium-236}-------->krypton+barium+3 neutron
Three fast neutrons are produced ,about 200Mev energy is released per fission event.Fission chain reaction occurs because of interactions between the neutrons and fissile isotope.The cahin reaction requires both the release of neutrons from the fissile isotopes undergoing nuclear fission and the subsequent absorption of some of these neutrons in fissile isotope.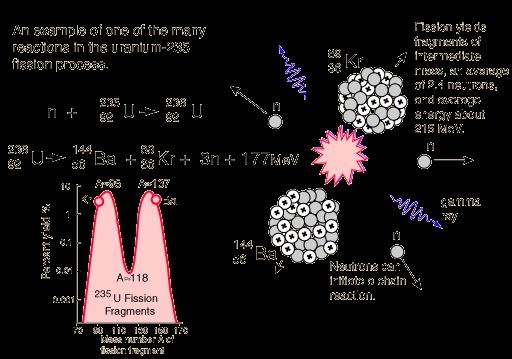
When an atom undergoes nuclear fission,a few neutrons are ejected from the reaction.These free neutrons will then interact with the surrounding medium and if more fissile fuel is present,some may be absorbed and cause more fissions.Thus the cycle repeats to give a reaction that is self-sustaining.
The minimum mass of sample required for self-sustained fission chain reaction is called critical mass.Fission of U-235 fives rise to the 3 fast neutrons,when slowed down,can cause three more U-235 nuclei to split,again 9 neutrons are produced which can caused the fission of more nuclei and so on.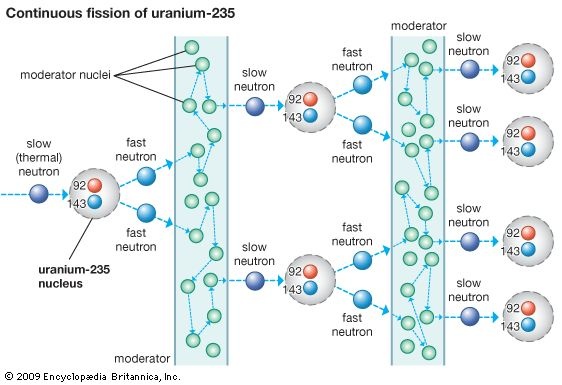 This process is said to be fission chain reaction,and it generates a tremendous amount of energy.
This process is said to be fission chain reaction,and it generates a tremendous amount of energy.