Quantum Numbers
Integral and half integral numbers which specify the state of a system or its components are known as quantum numbers.
TYPES:
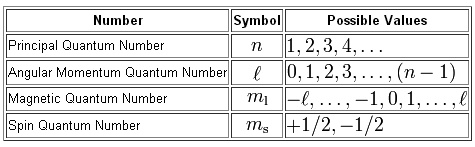
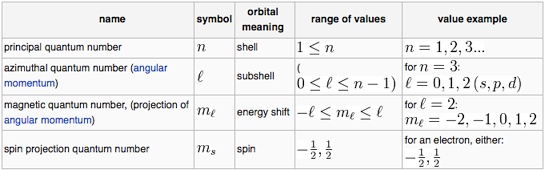
There are four types of quantum numbers which are as follows.
1) Principal or Total Quantum number
2) Azimuthal or Orbital Quantum number
3) Magnetic Quantum number
4) Spin Quantum number
Principal or Total Quantum number:
It characterized the main energy levels, and deals with the size of an atom.
It deals with the shells. It is denoted by ‘n’.
n = 1,2,3,………
alphabetically main energy levels are denoted by
K,L,M,N……..
Where K is the first main energy level, L is the 2nd, M is the 3rd and so on.
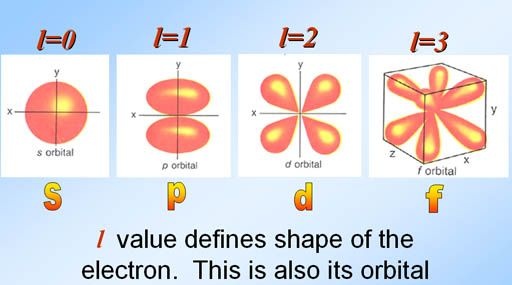
Azimuthal or Orbital Quantum number
Those numbers which describes the shape of an atom are called azimuthal or orbital quantum numbers.
It deals with sub-energy levels present in main energy levels that are called orbitals.
s ,p , d , f are the four sub-shells.
It is denoted by l.
Where l = n-1 where n = 1,2,3,……..
l is always zero or positive integer.
l = 0,1,2,3……
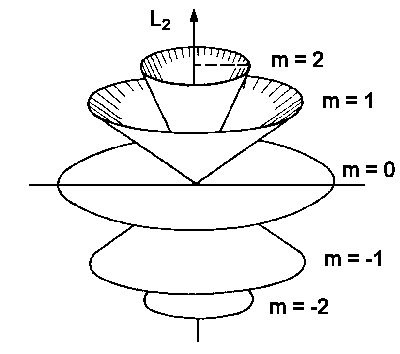
Magnetic Quantum number:
A Quantum number which determined the value of component of orbital angular momentum is known as magnetic quantum number.
It is denoted by ml.
The direction of l is described by ml.
ml = + and – (l)
ml = ……….-3,-2,-1,0,1,2,3………….
First three quantum numbers are related with the extrinsic property of an atom and these all shows that atom behaves as particle.
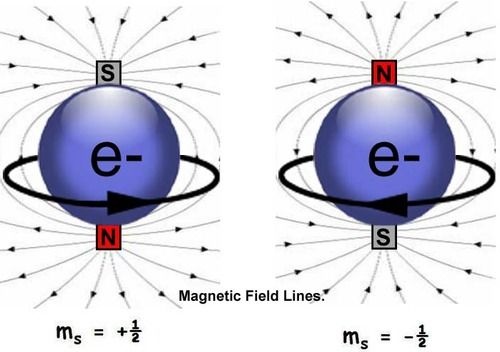
Spin Quantum number
The degree of freedom is known as spin.Quantum number associated with intrinsic degree of freedom is known as spin quantum number.
Spin can be parallel (spin up) or anti-parallel ( spin down)
It is denoted ny ms.
ms = + or – ½
for +1/2, the spin is parallel (spin up) and for -1/2 the spin is antiparallel (spin down).