Nitrogen in the represented by N. Its atomic number is 7 and atomic weight is 14. The melting point is -210 degree centigrade and boiling point is -196 degree centigrade. The covalent radius of nitrogen is 74 and ionic radius is 11 .

The ionization potential of nitrogen is 1398 and its density is 0.8042. Nitrogen must really exist in air.78% nitrogen is present in air on the outer atmosphere. It is also present in plants and animals body. There are so many methods for the preparation of nitrogen.

When ammonium nitrite is heated then nitrogen gas and water will be obtained. It also prepared by the resection of ammonia with cupric oxide as a result water copper and nitrogen gas is produced. Very small amount of nitrogen is soluble in water .

When nitrogen react with hydrogen at a temperature of 600 degree centigrade to form ammonia. Calcium carbide react with nitrogen at a very high temperature is 1000 degree centigrade to from carbon in solid form. Nitrogen react with oxygen to form nitrous oxide.

When nitrogen react with metal like lithium, magnesium and calcium to form nit rides .Nitrogen react with sodium carbonate in the presence of carbon to form sodium cyanide and carbon monoxide .
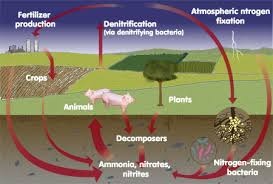
Nitrogen is used for the preparation of ammonia, nitric acid . It is also used in the industries for the manufacturing of different products .Nitrogen is used has laboratory reagent.



