Sigma-bond and its Formation
When two half filled orbitals overlap linearly in such way that the chances of finding the electrons around the line joining the two nuclei is maximum, then the formed bond is called sigma-bond.
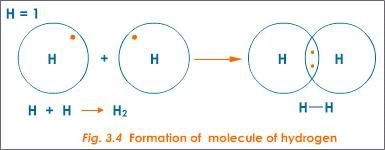
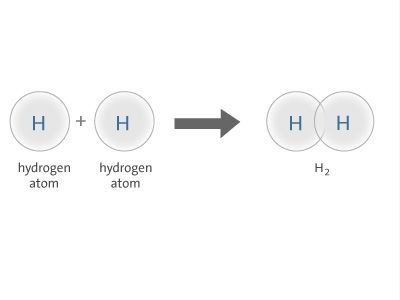
Examples of sigma bonds are given below:
H H , H F , F F
The S S orbitals overlap of two hydrogen atoms gives sigma-bond in hydrogen molecule.
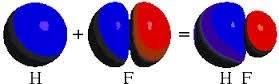
The S P orbitals overlap of hydrogen and fluorine atoms give sigma-bond in hydrogen fluoride.
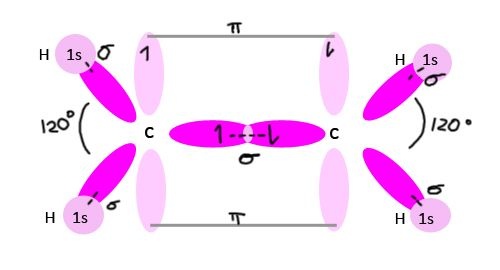
Pi-bond and its Formation
This bond is formed only when the two half filled co-planer p-orbitals overlapped in sideways manner such that the chances of finding the electrons is maximum perpendicular to the line joining the two nuclei is called a pi-bond. A pi-bond is formed only when the two atoms are already bonded by a sigma-bond.
Example of pi-bond in ethene is given below: