Metals and Alloys
Metal: Class of elements existing as oxide, carbonate, sulphide and phosphate compounds in natural rocks called ores.
Ferrous metals: metals in which iron (Fe) is the main constituent.
Non-Ferrous metals: metals in which iron is not the main constituent. Aluminum, copper.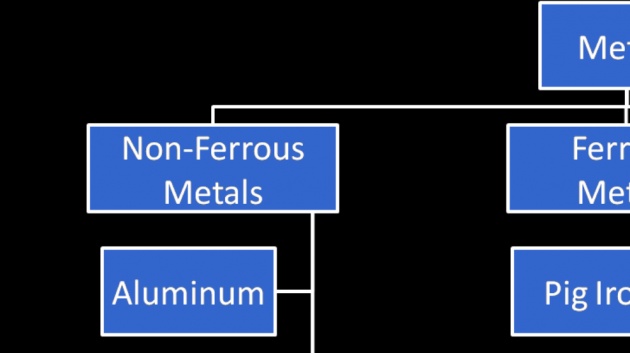
Iron
Iron is available in abundance, but it does not occur freely in nature. The iron content of main ores are:
Magnetite (Fe3O4) 70-75% iron
Haematite (Fe2O3) 70% iron
Limonite (2Fe2O3. 3H2O) 60% iron
Iron Pyrite (FeS) 47% iron
Siderite (FeCO3) 40% iron
Iron can combine with other elements and its properties is markedly altered and improved for varying conditions of service.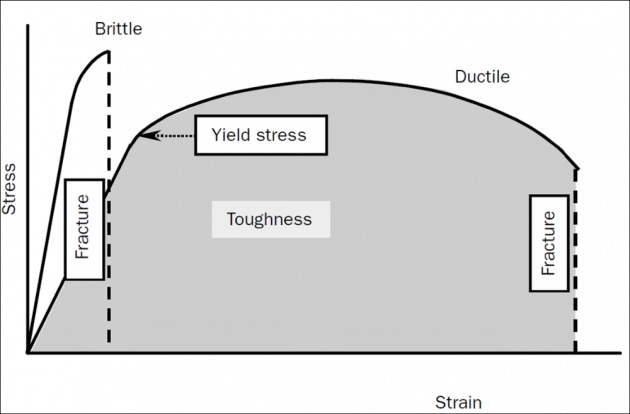
Fracture, brittle & ductile behavior
Fracture is the partitioning of materials into two or more pieces under the action of a static or slowly changing imposed load, at temperatures that are low compared with the melting temperature of the material.
Brittle fracture occurs when a material absorbs little or no energy prior to fracture.
Ductile fracture requires a material that can experience appreciable plastic (i.e. irreversible) deformation and energy absorption prior to fracture.
Hardening
Hardening involves heating a metal/steel and cooling rapidly (quenching) in a fluid etc
Hardening only the metal surface up to a depth of 1.5 mm
Process comprises of
Converting the outer skin to high carbon steel
Hardening the case and refining the core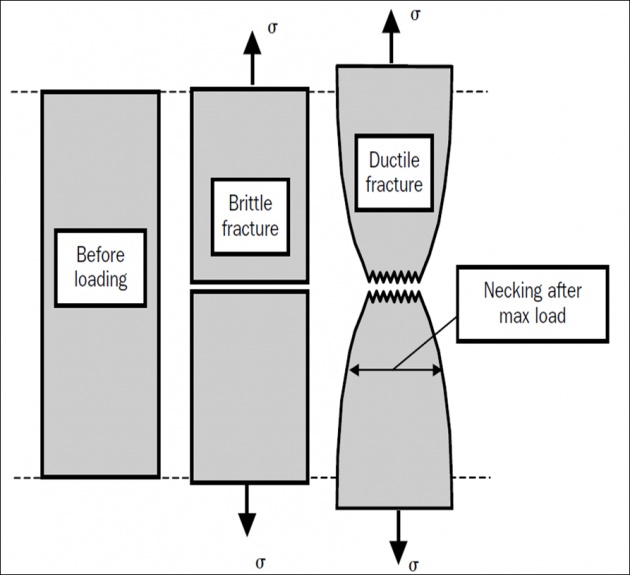
Tempering
To relieve the internal stresses and reduce brittleness, tempering is needed.
the steel is heated during the tempering process. Tempering is performed to improve ductility and toughness.
Pig iron
It is the basic material from which, wrought iron and steel are manufactured.
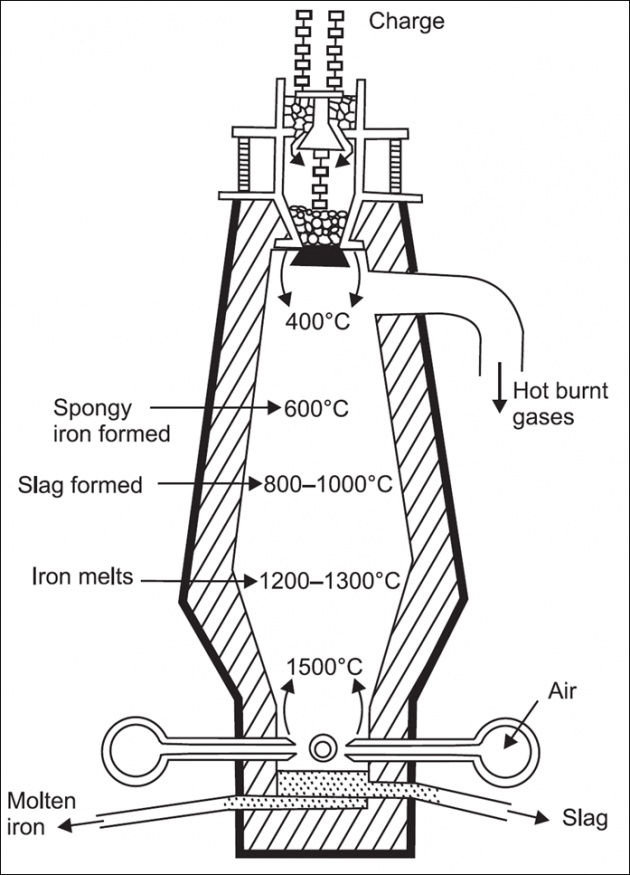
Iron ore is crushed to 50 mm size. Impurities are knocked off. Ore is calcined ( thermal treatment process in absence of air)to drive off moisture and then smelted in blast furnace.
Iron is deoxidized and limestone is added as flux to remove sulphur (Flux is a substance charged into blast furnace to lower the melting point of ore and to remove impurities such as ash, sulphur, etc).
The refined molten metal is tapped from furnace and cast in the form of bars called pigs. Hence the product is called pig iron.
Pig iron is the crudest form of iron. Pig iron contains 3-4 % carbon, 0.5-3.5 % silicon, 0.5-2 % manganese, 0.02-0.1 % sulphur and 0.03-1 % phosphorus.
Pig iron is hard and brittle with fusion temperature of 1200oC and melts easily.
Its compressive strength is high but is weak in tension and shear.
Pig iron does not rust.
It can not be riveted or welded.
It is most suitable for making columns, base plates, door brackets, etc.
Cast iron
Pig iron is re-melted with limestone (flux) and coke and poured into molds of desired shapes and sizes to get purer product called cast iron.
Methods of casting
Sand casting
Hollow casting
Vertical sand casting
Centrifugal casting
Die castin
Classification: grey, white, malleable, mottled, chilled and toughened cast iron
Uses: castings, rain water pipes, gutters, gratings, railings, cisterns, manhole covers and balustrades
Cast iron - properties
Coarse, crystalline and fibrous structure
Brittle, can not withstand shocks and impacts
Can not be welded or riveted
Can not be magnetized
Can be hardened but not tempered
Neither malleable (soft) nor ductile
Does not rust
Becomes soft in saline water
Fairly hard, not workable with hand file
Strong in compression, weak in tension and shear
Lacks plasticity, unsuitable for forging work
Wrought iron
All carbon and other elements in pig iron oxidized to obtain wrought iron
Carbon reduced below 0.25%
All impurities reduced below 0.5%
Uses
Roofing sheets, corrugated sheets, rods, gas and water pipes, boiler tubes, plain and ornamental iron work like grills, gates, railings, window guards, electromagnets
Wrought iron - properties
Fibrous structure with silky luster
Ductile and malleable
Tough, can withstand shocks and impacts
Neither be hardened nor tempered
Can be forged and welded
At 900°C two pieces can be joined by hammering. Melts at 1500°C
Rusts easily
Unaffected by saline water
Forms temporary magnets
Equally strong in tension, compression and shear
Specific gravity is 7.25
Steel
In steel the carbon content is in chemically combined form and may exist up to 1.5%
For a material to be classified as steel there should be no free graphite in its composition. Any free carbon makes it as cast iron
Steel categories
Dead mild steel less than 0.15% carbon
Mild, soft, low carbon steel 0.15 to 0.3% carbon
Medium carbon steel 0.3 to 0.8% carbon
High carbon steel 0.8 to 1.5% carbon
Mild steel
Steel with carbon content 0.15 to 0.3%
Called mild steel, low carbon steel or soft steel
Uses
Used in construction work as rolled sections, I-sec, T-sec, channels, angle irons, etc
MS round bars used in RCC as reinforcement
Plain and corrugated sheets as roofing
Used in manufacture of various tools, equipment, machine parts
Rail tracks, towers and industrial buildings
Fibrous structure with dark bluish color
Ductile and malleable
Tough and elastic than cast and wrought iron
More prone to rusting and corrodes easily
Can be permanently magnetized
Easily forged, welded and riveted
Withstands shocks and impacts
Not much affected by saline water
Equally strong in tension, compression and shear
Difficult to harden and temper
Specific gravity is 7.8
High carbon steel
Steel where in the carbon content is from 0.55 to 1.5%
Higher percentage of carbon renders it harder and tougher
Uses
Manufacture of tools like drills, files, chisels
Fine quality of cutlery
Parts of machines requiring to withstand shocks and vibrations
High carbon steel
Granular structure
Tough and elastic than mild steel
Easier to harden and temper
More difficult to forge, weld and rivet
Can be permanently magnetized
Strong in compression than tension and shear Strong in compression than tension and shear
Withstands shocks and impacts
High tension steel
Low carbon steel with carbon nearly 0.15%
Also called high strength steel
Less weight is required due to increased tensile strength
Withstands atmospheric erosion
Tougher and more elastic
More brittle and less ductile
Extensively used in reinforcing prestressed concrete structures
Reinforcing Steel
Mild steel or high tension steel is embedded as reinforcement in plain cement concrete to provide tensile strength
Flat, square and round bars used
Welded wire mesh also used as reinforcement
Alloy steels
Stainless Steel
Nickel Steel
Vanadium Steel
Tungsten Steel
Manganese Steel
Stainless steel
Structural steel with copper content of 0.2% resists atmospheric corrosion better than structural steel with no copper
Chromium is most effective ingredient for corrosion resistance. Corrosion protection is due to dense film of oxide formed over metal surface.
Steel with chromium over 16% called as stainless steel
Group-1
Chromium less than 16% and carbon less than 0.4%. Respond to heat treatment, are not brittle, can be machined and welded. Resist weather and water
Group-2
Chromium higher than 16% and carbon less than 0.4%. Do not respond to heat treatment, are brittle. Can be forged, rolled, cold drawn and machined. Can be welded and resist corrosion.
Group-3
Sufficient chromium to make it non-magnetic. Very tough and do not respond to heat treatment. Can be forged, rolled, cold drawn but machined with difficulty.
Nickel steel
Contains 0.5 to 1.0% carbon and 3.5% nickel which imparts hardness, toughness, strength and reduces rust formation
Used in manufacture of automobile parts, airplane parts, cables and propeller shafts.
Steel with high nickel content (30 to 40%) is called invar, with very low coefficient of thermal expansion, and is used to make delicate instruments
Vanadium steel
Contains 0.1 to 2.0% vanadium
Very strong and ductile
Capable of resisting shocks
High elastic limit
Tungsten steel
Contains 14 to 20% tungsten, 3 to 8% chromium and very small amount of carbon, vanadium and molybdenum
Also called high speed steel
Hardens at high temperature and retains temper
Used for making drilling machines and high speed cutting tools
Manganese steel
Contains 12 to 15% manganese
Very hard, tough and non-magnetic
Used for making machine parts and points and crossings in rail tracks
Preservation of steel
Rusting: Oxidation of iron at the surface, which is activated by presence of moisture and carbon dioxide and accelerated by atmospheric pollution
Iron → ferrous bicarbonate → ferric bicarbonate → hydrated ferric oxide
Corrosion: Phenomenon of slow but steady eating away of metal due to rust formation
Tarring: dipping of iron in hot coal tar to form a film on metal. Pipes or pole ends
Painting: application of lead paints on exposed metal surfaces like roof trusses, bridge structure, etc
Enameling: smaller surfaces treated with enamel
Galvanizing: depositing a fine film of zinc or iron surface
Sheradizing: acid solution washed metal surface is covered with zinc dust and heated in furnace to form a thin layer of molten zinc
Tin plating: dipping in bath of molten tin
Electroplating: depositing a thin film of nickel, chromium, cadmium, copper or zinc by the electrolysis process. Metal surface is cathode and deposition metal is anode



