Malaria is a mosquito-borne infectious disease of humans and other animals caused by parasitic protozoans (a group of single-celled microorganisms) belonging to the genus Plasmodium.[1] Malaria causes symptoms that typically include fever, fatigue,vomiting, and headaches. In severe cases it can cause yellow skin, seizures, coma or death. The disease is transmitted by the biting of mosquitos, and the symptoms usually begin ten to fifteen days after being bitten. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.
The disease is most commonly transmitted by an infected female Anopheles mosquito. The mosquito bite introduces the parasitesfrom the mosquito's saliva into a person's blood. The parasites travel to the liver where they mature and reproduce. Five species of Plasmodium can infect and be spread by humans. Most deaths are caused by P. falciparum because P. vivax, P. ovale, andP. malariae generally cause a milder form of malaria. The species P. knowlesi rarely causes disease in humans. Malaria is typically diagnosed by the microscopic examination of blood using blood films, or with antigen-based rapid diagnostic tests.Methods that use the polymerase chain reaction to detect the parasite's DNA have been developed, but are not widely used in areas where malaria is common due to their cost and complexity.
The risk of disease can be reduced by preventing mosquito bites by using mosquito nets and insect repellents, or with mosquito-control measures such as spraying insecticides and draining standing water.several medications are available to prevent malaria in travellers to areas where the disease is common. Occasional doses of the medication sulfadoxine/pyrimethamine are recommended in infants and after the first trimester of pregnancy in areas with high rates of malaria. Despite a need, no effectivevaccine exists, although efforts to develop one are ongoing. The recommended treatment for malaria is a combination ofantimalarial medications that includes an artemisinin. The second medication may be either mefloquine, lumefantrine, or sulfadoxine/pyrimethamine. Quinine along with doxycycline may be used if an artemisinin is not available. It is recommended that in areas where the disease is common, malaria is confirmed if possible before treatment is started due to concerns of increasing drug resistance. Resistance among the parasites has developed to several antimalarial medications; for example,chloroquine-resistant P. falciparum has spread to most malarial areas, and resistance to artemisinin has become a problem in some parts of Southeast Asia.
The disease is widespread in the tropical and subtropical regions that exist in a broad band around the equator. This includes much of Sub-Saharan Africa, Asia, and Latin America. Malaria is commonly associated with poverty and has a major negative effect on economic development. In Africa, it is estimated to result in losses of US$12 billion a year due to increased healthcare costs, lost ability to work, and negative effects on tourism. The World Health Organization reports there were 198 million cases of malaria worldwide in 2013. This resulted in an estimated 584,000 to 855,000 deaths, the majority (90%) of which occurred in Africa.
Signs and symptoms
The signs and symptoms of malaria typically begin 8–25 days following infection; however, symptoms may occur later in those who have taken antimalarial medications as prevention. Initial manifestations of the disease—common to all malaria species—are similar to flu-like symptoms, and can resemble other conditions such as sepsis, gastroenteritis, and viral diseases. The presentation may include headache, fever, shivering, joint pain, vomiting, hemolytic anemia, jaundice, hemoglobin in the urine,retinal damage, and convulsions.
The classic symptom of malaria is paroxysm—a cyclical occurrence of sudden coldness followed by shivering and then fever and sweating, occurring every two days (tertian fever) in P. vivax and P. ovale infections, and every three days (quartan fever) forP. malariae. P. falciparum infection can cause recurrent fever every 36–48 hours, or a less pronounced and almost continuous fever.
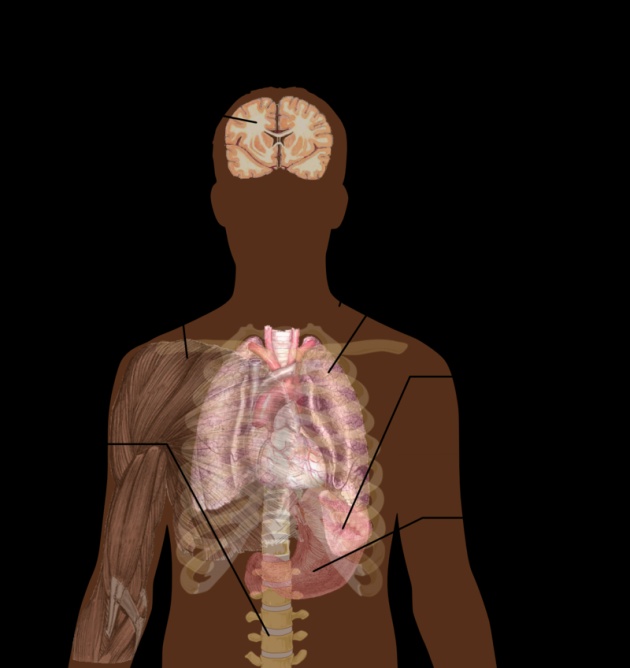
Severe malaria is usually caused by P. falciparum (often referred to as falciparum malaria). Symptoms of falciparum malaria arise 9–30 days after infection. Individuals with cerebral malaria frequently exhibit neurological symptoms, including abnormal posturing, nystagmus, conjugate gaze palsy (failure of the eyes to turn together in the same direction), opisthotonus, seizures, orcoma
Cause
Malaria parasites belong to the genus Plasmodium (phylum Apicomplexa). In humans, malaria is caused by P. falciparum, P. malariae, P. ovale, P. vivax and P. knowlesi. Among those infected, P. falciparum is the most common species identified (~75%) followed by P. vivax (~20%). Although P. falciparum traditionally accounts for the majority of deaths, recent evidence suggests that P. vivax malaria is associated with potentially life-threatening conditions about as often as with a diagnosis of P. falciparum infection. P. vivax proportionally is more common outside Africa. There have been documented human infections with several species of Plasmodium from higher apes; however, except for P. knowlesi—a zoonotic species that causes malaria in macaques....these are mostly of limited public health importance.
Global warming is likely to affect malaria transmission, but the severity and geographic distribution of such effects is uncertain.
Life cycle
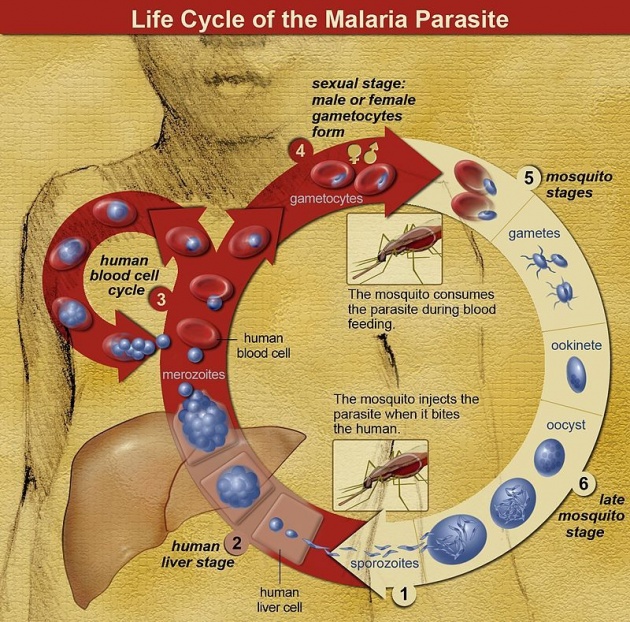
In the life cycle of Plasmodium, a female Anopheles mosquito (the definitive host) transmits a motile infective form (called the sporozoite) to a vertebrate host such as a human (the secondary host), thus acting as a transmissionvector. A sporozoite travels through the blood vessels to liver cells (hepatocytes), where it reproduces asexually(tissue schizogony), producing thousands of merozoites. These infect new red blood cells and initiate a series of asexual multiplication cycles (blood schizogony) that produce 8 to 24 new infective merozoites, at which point the cells burst and the infective cycle begins anew.
Other merozoites develop into immature gametocytes, which are the precursors of male and female gametes. When a fertilised mosquito bites an infected person, gametocytes are taken up with the blood and mature in the mosquito gut. The male and female gametocytes fuse and form an ookinete—a fertilized, motile zygote. Ookinetes develop into new sporozoites that migrate to the insect's salivary glands, ready to infect a new vertebrate host. The sporozoites are injected into the skin, in the saliva, when the mosquito takes a subsequent blood meal.
Only female mosquitoes feed on blood; male mosquitoes feed on plant nectar, and do not transmit the disease. The females of the Anopheles genus of mosquito prefer to feed at night. They usually start searching for a meal at dusk, and will continue throughout the night until taking a meal. Malaria parasites can also be transmitted byblood transfusions, although this is rare.
Owing to the non-specific nature of the presentation of symptoms, diagnosis of malaria in non-endemic areas requires a high degree of suspicion, which might be elicited by any of the following: recent travel history, enlarged spleen, fever, low number of platelets in the blood, and higher-than-normal levels of bilirubin in the blood combined with a normal level of white blood cells.
Malaria is usually confirmed by the microscopic examination of blood films or by antigen-based rapid diagnostic tests (RDT).Microscopy is the most commonly used method to detect the malarial parasite—about 165 million blood films were examined for malaria in 2010. Despite its widespread usage, diagnosis by microscopy suffers from two main drawbacks: many settings (especially rural) are not equipped to perform the test, and the accuracy of the results depends on both the skill of the person examining the blood film and the levels of the parasite in the blood. The sensitivity of blood films ranges from 75–90% in optimum conditions, to as low as 50%. Commercially available RDTs are often more accurate than blood films at predicting the presence of malaria parasites, but they are widely variable in diagnostic sensitivity and specificity depending on manufacturer, and are unable to tell how many parasites are present.
In regions where laboratory tests are readily available, malaria should be suspected, and tested for, in any unwell person who has been in an area where malaria is endemic. In areas that cannot afford laboratory diagnostic tests, it has become common to use only a history of fever as the indication to treat for malaria—thus the common teaching "fever equals malaria unless proven otherwise". A drawback of this practice is overdiagnosis of malaria and mismanagement of non-malarial fever, which wastes limited resources, erodes confidence in the health care system, and contributes to drug resistance. Although polymerase chain reaction-based tests have been developed, they are not widely used in areas where malaria is common as of 2012, due to their complexity.
Medication
Malaria prophylaxis
There are a number of drugs that can help prevent or interrupt malaria in travelers to places where infection is common. Many of these drugs are also used in treatment.Chloroquine may be used where chloroquine-resistant parasites are not common. In places where Plasmodium is resistant to one or more medications, three medications—mefloquine (Lariam), doxycycline (available generically), or the combination of atovaquone and proguanil hydrochloride (Malarone)—are frequently used when prophylaxis is needed. Doxycycline and the atovaquone plus proguanil combination are the best tolerated; mefloquine is associated with death, suicide, and neurological and psychiatric symptoms.
The protective effect does not begin immediately, and people visiting areas where malaria exists usually start taking the drugs one to two weeks before arriving and continue taking them for four weeks after leaving (except for atovaquone/proguanil, which only needs to be started two days before and continued for seven days afterward). The use of preventative drugs is often not practical for those who live in areas where malaria exists, and their use is usually only in pregnant women and short-term visitors. This is due to the cost of the drugs, side effects from long-term use, and the difficulty in obtaining anti-malarial drugs outside of wealthy nations. During pregnancy, medication to prevent malaria has been found to improve the weight of the baby at birth and decrease the risk of anemia in the mother. The use of preventative drugs where malaria-bearing mosquitoes are present may encourage the development of partial resistance
History
History of malaria and Mosquito-malaria theory
Although the parasite responsible for P. falciparum malaria has been in existence for 50,000–100,000 years, the population size of the parasite did not increase until about 10,000 years ago, concurrently with advances in agriculture and the development of human settlements. Close relatives of the human malaria parasites remain common in chimpanzees. Some evidence suggests that the P. falciparum malaria may have originated in gorillas.
References to the unique periodic fevers of malaria are found throughout recorded history, beginning in 2700 BC in China. Hippocrates described periodic fevers, labelling them tertian, quartan, subtertian and quotidian. The Roman Columella associated the disease with insects from swamps. Malaria may have contributed to the decline of the Roman Empire, and was so pervasive in Rome that it was known as the "Roman fever". Several regions in ancient Rome were considered at-risk for the disease because of the favourable conditions present for malaria vectors. This included areas such as southern Italy, the island of Sardinia, the Pontine Marshes, the lower regions of coastal Etruria and the city of Rome along the Tiber River. The presence of stagnant water in these places was preferred by mosquitoes for breeding grounds. Irrigated gardens, swamp-like grounds, runoff from agriculture, and drainage problems from road construction led to the increase of standing water.
In April 1894, a Scottish physician Sir Ronald Ross visited Sir Patrick Manson at his house on Queen Anne Street, London. This visit was the start of four years of collaboration and fervent research that culminated in 1898 when Ross, who was working in the Presidency General Hospital in Calcutta, proved the complete life-cycle of the malaria parasite in mosquitoes. He thus proved that the mosquito was the vector for malaria in humans by showing that certain mosquito species transmit malaria to birds. He isolated malaria parasites from the salivary glands of mosquitoes that had fed on infected birds. For this work, Ross received the 1902 Nobel Prize in Medicine. After resigning from the Indian Medical Service, Ross worked at the newly established Liverpool School of Tropical Medicine and directed malaria-control efforts in Egypt, Panama, Greece and Mauritius. The findings of Finlay and Ross were later confirmed by a medical board headed by Walter Reed in 1900. Its recommendations were implemented by William C. Gorgas in the health measures undertaken during construction of the Panama Canal. This public-health work saved the lives of thousands of workers and helped develop the methods used in future public-health campaigns against the disease.
The first effective treatment for malaria came from the bark of cinchona tree, which contains quinine. This tree grows on the slopes of the Andes, mainly in Peru. The indigenous peoples of Peru made a tincture of cinchona to control fever. Its effectiveness against malaria was found and the Jesuits introduced the treatment to Europe around 1640; by 1677, it was included in the London Pharmacopoeia as an antimalarial treatment. It was not until 1820 that the active ingredient, quinine, was extracted from the bark, isolated and named by the French chemists Pierre Joseph Pelletier and Joseph Bienaimé Caventou.
Quinine became the predominant malarial medication until the 1920s, when other medications began to be developed. In the 1940s, chloroquine replaced quinine as the treatment of both uncomplicated and severe malaria until resistance supervened, first in Southeast Asia and South America in the 1950s and then globally in the 1980s.
The medicinal value of Artemisia annua has been used by Chinese herbalists in traditional Chinese medicines for 2,000 years. In 1596, Li Shizhen recommended tea made from qinghao specifically to treat malaria symptoms in his "Compendium of Materia Medica". Artemisinins, discovered by Chinese scientistTu Youyou and colleagues in the 1970s from the plant Artemisia annua, became the recommended treatment for P. falciparum malaria, administered in combination with other antimalarials as well as in severe disease. Tu says she was influenced by a traditional Chinese herbal medicine source, The Handbook of Prescriptions for Emergency Treatments, written in 340 by Ge Hong For her work on malaria, Tu Youyou received the 2015 Nobel Prize in Physiology or Medicine
Plasmodium vivax was used between 1917 and the 1940s for malariotherapy—deliberate injection of malaria parasites to induce fever to combat certain diseases such as tertiary syphilis. In 1927, the inventor of this technique, Julius Wagner-Jauregg, received the Nobel Prize in Physiology or Medicine for his discoveries. The technique was dangerous, killing about 15% of patients, so it is no longer in use.
Vaccine
Immunity (or, more accurately, tolerance) to P. falciparum malaria does occur naturally, but only in response to years of repeated infection. An individual can be protected from a P. falciparum infection if they receive about a thousand bites from mosquitoes that carry a version of the parasite rendered non-infective by a dose of X-rayirradiation. An effective vaccine is not yet available for malaria, although several are under development. The highly polymorphic nature of many P. falciparum proteins results in significant challenges to vaccine design. Vaccine candidates that target antigens on gametes, zygotes, or ookinetes in the mosquito midgut aim to block the transmission of malaria. These transmission-blocking vaccines induce antibodies in the human blood; when a mosquito takes a blood meal from a protected individual, these antibodies prevent the parasite from completing its development in the mosquito. Other vaccine candidates, targeting the blood-stage of the parasite's life cycle, have been inadequate on their own. For example, SPf66 was tested extensively in areas where the disease is common in the 1990s, but trials showed it to be insufficiently effective.Several potential vaccines targeting the pre-erythrocytic stage of the parasite's life cycle are being developed, with RTS,S as a leading candidate; it is expected to be licensed in 2015. A US biotech company, Sanaria, is developing a pre-erythrocytic attenuated vaccine called PfSPZ that uses whole sporozoites to induce an immune response. In 2006, the Malaria Vaccine Advisory Committee to the WHO outlined a "Malaria Vaccine Technology Roadmap" that has as one of its landmark objectives to "develop and license a first-generation malaria vaccine that has a protective efficacy of more than 50% against severe disease and death and lasts longer than one year" by 2015.