Experiment
Apparatus
Wooden box with moving lamp inside the box and photocell at one end of the box, ammeter etc.
Procedure
Arrange the apparatus as shown, basic theme of arrangement:
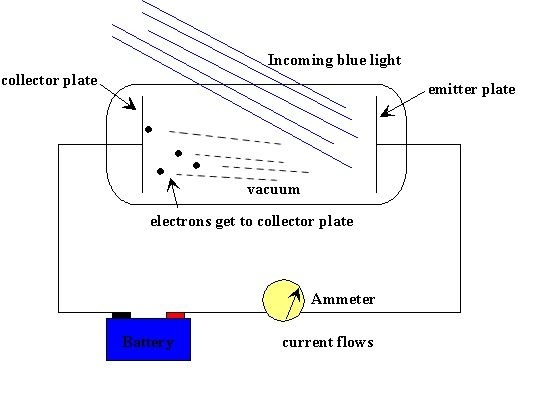
Switch on the lamp, slide the lamp, along the length of the box, increasing or decreasing the intensity of light on photocell. Observe the current at each specific distance of the lamp from the photocell. We know, intensity of light is inversely proportional to the square of the distance from the light source, i.e.
Intensity is proportional to 1/r^2
The photoelectric current, i.e. deflection in an ammeter, is directly proportional to the intensity of light.
Deflection is proportional to intensity
Therefore, photo current I,
I is proportional to 1/r^2
Precautions
- Never expose the cell for a longer period of time to light.
- Move the lamp gradually.
- Take the measurements carefully.
- The angle of incidence of light must not be changed on photocell.
Observations and graphical visualization of the data obtained
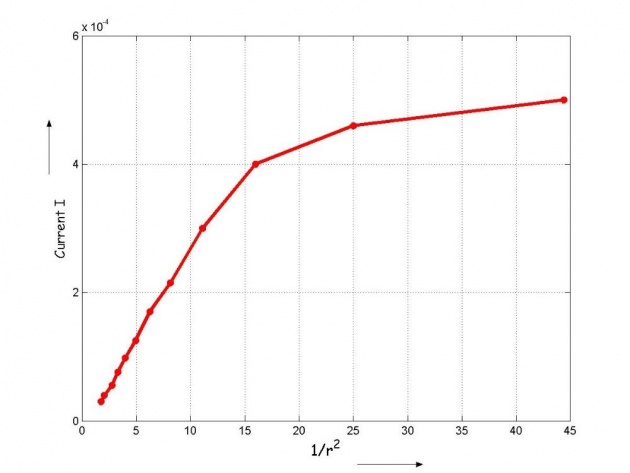
Conclusion
From the graph we see the direct relation between current conducted by the emission of electrons when light falls on the photocell and reciprocal of the distance between the light source and the photocell. In other terms Current I is directly proportional to the intensity of light because,
Intensity is proportional to 1/r^2
The intensity, when increases the chances of ejection of more electrons increases. The intensity only affects the number of electrons to be ejected that is why current is increasing with increasing intensity.
The graph is not a perfect straight line, this may be due to some inaccuracy of the measuring instruments but up to an extent it shows the direct relation between the intensity of light and photoelectric current. In the graph, even for higher values of intensity the current increases gradually. This may be due to the fact that the maximum of the surface electrons from the cell are emitted.
Now if the more energetic radiation falls upon the cell, the current probably would become directly proportional to intensity again. The energy of the radiation depends upon its frequency not on intensity. The radiation with high frequency would impart more energy to the electron and can penetrate deep inside also, to excite the electrons.



