The very high energy rays which are produced by colliding fast moving electrons with heavy metal anode in the discharge tube are called x-rays.Roentgen had discovered these rays in 1895.The figure which show the production of these rays is given below. First of all the electron emit from cathode and then they collide with a heavy metal target known as anti-cathode and give out x-ray.
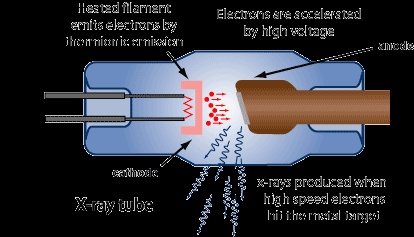
The x-rays emit from target in all directions but through aluminum window only a small portion of these rays is passed.When these rays fall on crystals of potassium ferro cyanide,they show diffraction.They give line spectrum on photo graphic plates after the diffraction.The spectrum of x-rays consist of K-series,L-series and M-series.Each series consist of lines such as K_A,K_B,L_A,L_B etc.These rays are electromagnetic rays.X-rays of each metal are different the wavelength and frequency depeds on atomic number of metal target.
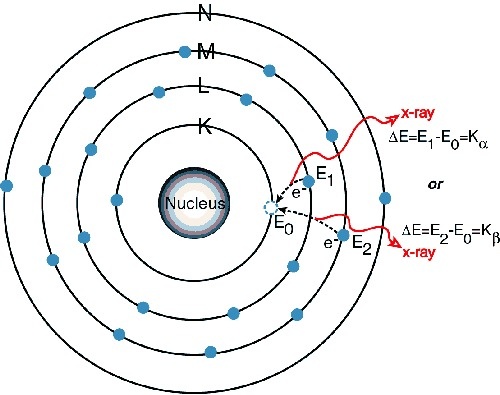
Moseley studed these rays in a systematic and comprehensive way in 1913-1914.Thirty eight different metal from aluminium to gold were used by Moseley used it as target metal in the x-rays tube.He produced x-rays having wavelength ranges from 0.04 to 8A^0.Finally following results were concluded by Moseley.
The spectrum of x-rays consist of tow distinct group the rays called K-series have shorter wavelength and x-rays are called L-series have longer wavelength.If atomic number of a target element is lesser then the wavelength of x-rays will be longer.Moseley law states that the frequency of these rays is directly proportional to the square of atomic number of target metal.Moseley law states that it is atomic number which determined the physical and chemical properties of an element.